Applicant Guide
Canadian Consortium on Neurodegeneration in Aging (CCNA) Phase III – Research Teams
Table of Contents
- 1. Introduction
- 2. CCNA Central Research Support
- 3. CCNA Data and Biological Samples
- 4. Contact Information
- Appendix A: COMPASS-ND Baseline Data
- Appendix B: COMPASS-ND Fluid Biomarker Data
1. Introduction
As specified in the "Canadian Consortium on Neurodegeneration in Aging (CCNA) Phase III: Research Teams" funding opportunity, this Applicant Guide is the sole source of information for applicant teams to learn more about the CCNA Operations Centre, including its Central Research Support and Data and Biological Samples holdings.
The Applicant Guide will be the only source of information used by peer reviewers to assess feasibility of planned research projects and associated activities as it relates to the CCNA Operations Centre. Applicants are encouraged to reference the information (section and page number) found in this applicant guide in their applications to the Canadian Institutes of Health Research (CIHR), as applicable.
Please Note: Information acquired from other online sources related to the CCNA may be outdated and should not be used to develop applications. In addition, applicant teams must not communicate directly with the CCNA Operations Centre in developing applications for this funding opportunity. All inquiries should be directed to the CIHR Contact Centre (support-soutien@cihr-irsc.gc.ca or 1-888-603-4178).
Disclaimer: Information included in this Applicant Guide was developed by the CCNA Operations Centre in collaboration with CIHR. CIHR is not responsible for any errors, omissions, or for the results obtained from the use of this information.
2. CCNA Central Research Support
In CCNA Phase III, the creation of a Central Research Support was a significant milestone aimed at catalyzing and supporting research efforts across various domains. Central Research Support comprises several specialized programs designed to provide comprehensive guidance, resources, and collaboration opportunities to enhance the quality and impact of dementia research.
These programs include:
- Knowledge Mobilization
- Training and Capacity Building
- Indigenous Cognitive Health Program
- The Engagement of People with Lived Experience of Dementia
- Women Sex Gender and Dementia
These programs will be available to support successful CCNA Phase III research teams and cannot be accessed during the development of your application.
2.1 Knowledge Mobilization (KM)
The Knowledge Mobilization (KM) program will be available to provide consultation and guidance on:
- Expanding and refining the project's KM plan including key messages, KM goals, and KM products that will support the goals.
- Tailoring KM products to specific audiences, for example, adapting scientific language to plain language.
- Disseminating KM products, including leveraging CCNA partnerships, when appropriate.
- Engaging relevant knowledge users throughout the project.
- Evaluating the achievement of KM goals.
- Using relevant evidence from the KM scientific literature and KM tools.
2.2 Training and Capacity Building (TCB)
The Training and Capacity Building (TCB) program will:
- Maintain an online resource library for trainees to access training for developing skills related to the logistics of conducting research, how to prepare research proposals, how to prepare and build a successful grant application, budgeting, research standards, and ethics submissions.
- Act as a central research support for trainees on best practices regarding how to access and work with the Comprehensive Assessment of Neurodegeneration and Dementia Study (COMPASS-ND) study data using the Longitudinal Online Research and Imaging System (LORIS) platform.
- Foster networking and career development of trainees through networking opportunities such as the CCNA's annual scientific meeting - "Science Days and Partners Forum."
- Provide interdisciplinary training and capacity building by continuing to build our existing CCNA Investigator Member database, connecting mentors and mentees for one-year mentorship experiences. Investigators in Phase III will be invited and strongly encouraged to join our mentor pool and may be matched with mentees based on alignment of: (i) mentors' experiences, areas of expertise, and research interests, with (ii) mentees' research interests, career stage and goals, and identified areas of growth.
2.3 Indigenous Cognitive Health Program (ICH)
The Indigenous Cognitive Health (ICH) program will be available to provide consultation and guidance on:
- Indigenous data access and publication requests.
- Capacity building for Indigenous-led and Indigenous-informed research.
- Capacity building for Indigenous-led and Indigenous-informed knowledge mobilization.
2.4 The Engagement of People with Lived Experience of Dementia (EPLED)
The Engagement of People with Lived Experience of Dementia (EPLED) program works to enable those with lived experience of dementia (i.e., people living with dementia, caregivers/care partners, friends and family) to be meaningfully and actively involved in research. The EPLED program will be available to provide consultation and guidance to Research Teams on engaging people with lived experience of dementia in their proposed research. The EPLED program will also facilitate engagement activities in the proposed research, if the research aligns with the interests of the EPLED Advisory Group or its individual members.
2.5 Women Sex Gender and Dementia (WSGD)
The Women Sex Gender and Dementia (WSGD) program will be available to provide consultation and guidance on:
- Incorporation of sex and gender into research design for specific projects.
- Determining the sex and gender knowledge and knowledge needs of dementia researchers.
- Using sex and gender methods and measures through webinars/talks/access to resources.
- Mining the COMPASS-ND with a sex and gender lens.
- Considerations of inclusion, diversity, equity, accessibility, and intersectionality in dementia research.
- Inclusion of sex and gender in programs and publications in professional societies.
- Evaluating the achievement of the incorporation of sex and gender in dementia research.
3. CCNA Data and Biological Samples
3.1 Overview
The Comprehensive Assessment of Neurodegeneration and Dementia (COMPASS-ND) is the clinical cohort study of CCNA. This unique cohort study includes a wide range of neurodegenerative conditions to enable comparisons across disease states and the identification of common and unique factors across these diseases. The Initial Assessment of COMPASS-ND comprises a wealth of data collected from over 1,150 participants across 32 testing sites and spans 10 syndrome cohorts and a control group. See below for a demographic overview of the participant groups.
| Cohort | Diagnosis | Tally | Women | Education (mean, in years) | Age (mean, in years) |
|---|---|---|---|---|---|
| CU | Cognitively unimpaired | 176 | 62.5% | 15.6 | 69.9 |
| SCI | Subjective cognitive impairment | 149 | 65.8% | 15.9 | 69.9 |
| MCI | Mild cognitive impairment (MCI) | 280 | 37.5% | 15 | 71.2 |
| V-MCI | MCI with silent vascular lesions | 152 | 47.4% | 14.6 | 76.2 |
| AD | Alzheimer's disease | 113 | 44.2% | 15 | 72.9 |
| Mixed | Mixed dementia | 87 | 48.3% | 14.9 | 77.3 |
| FTD | Frontotemporal dementia | 40 | 45.0% | 14.6 | 64.3 |
| PD | Parkinson's disease | 82 | 45.1% | 15.8 | 66.8 |
| PD-MCI | Parkinson's disease with MCI | 45 | 15.6% | 15.4 | 71.3 |
| PDD | Parkinson's disease dementia | 16 | 6.3% | 15.9 | 76.9 |
| LBD | Lewy body disease | 32 | 6.3% | 14.7 | 72.8 |
| Other | Other dementia | 2 | 0.0% | 15 | 78.8 |
| Overall | 1,173 | 46.5% | 15.2 | 71.9 | |
| Age | Years |
|---|---|
| Minimum | 42.1 |
| Maximum | 91 |
| Mean | 71.9 |
| Education | Years |
|---|---|
| Minimum | 3 |
| Maximum | 23 |
| Mean | 15.2 |
| Testing language | Percent |
|---|---|
| English | 89.3% |
| French/bilingual | 10.7% |
| Community | Percent |
|---|---|
| Urban | 59.0% |
| Suburban | 30.7% |
| Rural | 10.3% |
| Ethnicity | Percent |
|---|---|
| White | 92.1% |
| Visible minority | 7.9% |
New Demographic overview of the caregiving assessment data in COMPASS-ND (Updated: 2024-11-14)
The following tables provide a demographic overview of the caregiving assessment data in COMPASS-ND; both participants and/or their respective primary informants may report being caregivers.
Participants
| Cohort | Diagnosis | Number of caregivers | Female (%) | Education (mean, in years) | Age (mean, in years) |
|---|---|---|---|---|---|
| CU | Cognitively unimpaired | 52 | 61.5% | 15.2 | 69.3 |
| SCI | Subjective cognitive impairment | 58 | 67.2% | 16 | 69.2 |
| MCI | Mild cognitive impairment (MCI) | 92 | 38% | 15 | 70.7 |
| V-MCI | MCI with silent vascular lesions | 40 | 32.5% | 14.2 | 75.9 |
| AD | Alzheimer's disease | 21 | 33.3% | 16.1 | 73.1 |
| Mixed | Mixed dementia | 18 | 55.6% | 14.4 | 75.5 |
| FTD | Frontotemporal dementia | 12 | 50% | 13.7 | 66.9 |
| PD | Parkinson's disease | 21 | 42.9% | 16.8 | 67.1 |
| PD-MCI | Parkinson's disease with MCI | 11 | 18.2% | 16.2 | 68.9 |
| PDD | Parkinson's disease dementia | 2 | 0% | 13 | 82.7 |
| LBD | Lewy body disease | 3 | 33.3% | 13 | 78.9 |
| Overall | 330 | 46.7% | 15.2 | 70.9 | |
Primary Informant (an individual who has regular and weekly interactions with the participant)
| Cohort | Number of caregivers | Female (%) | Education (mean, in years) | Age (mean, in years) |
|---|---|---|---|---|
| Primary informant | 500 | 68% | 15.2 | 70 |
All Caregivers in the COMPASS-ND Study
| Cohort | Number of caregivers | Female (%) | Education (mean, in years) | Age (mean, in years) |
|---|---|---|---|---|
| Participant | 330 | 46.7% | 15.2 | 70.9 |
| Primary informant | 500 | 68% | 15.2 | 70 |
| Overall | 830 | 57.4% | 15.2 | 70.5 |
| Age | Years |
|---|---|
| Minimum | 29 |
| Maximum | 94 |
| Mean | 70.5 |
| Education | Years |
|---|---|
| Minimum | 4 |
| Maximum | 23 |
| Mean | 15.2 |
3.1.1 Data Collected
Collected data include extensive clinical, cognitive, and neuropsychological measures, as well as objective sensory measures, clinically verified diagnoses, psychosocial and neuropsychiatric measures, and study partner questionnaires. In addition to alphanumeric data, the study also captures standardized 3T neuroimaging, recorded speech, and biological samples that yielded plasma, serum, and cerebrospinal fluid (CSF) biomarkers as well as genetic data. Saliva samples collected cohort-wide are currently undergoing microbiome analyses, and these results are currently not available.
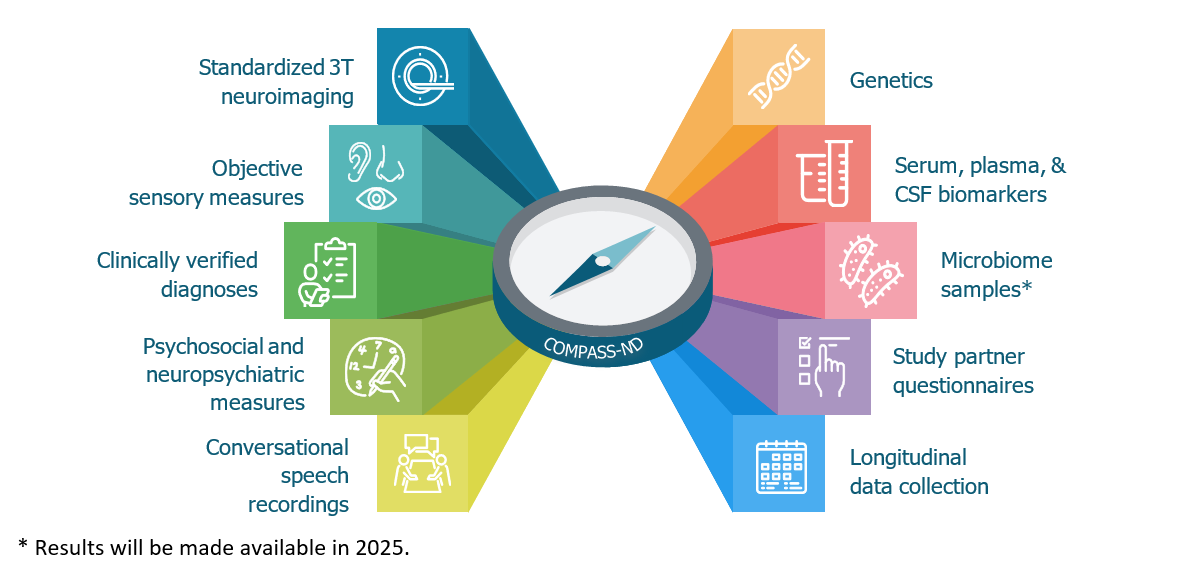
Figure 1 long description
The COMPASS-ND data include:
- Standardized 3T neuroimaging
- Objective sensory measures
- Clinically verified diagnoses
- Psychosocial and neuropsychiatric measures
- Conversational speech recordings
- Genetics
- Serum, plasma, & CSF biomarkers
- Microbiome samples (Results will be made available in 2025)
- Study partner questionnaires
- Longitudinal data collection
The deeply-phenotyped data are collected over multiple, in-person visits; together, these visits capture the Initial (Baseline) data for the study. A list of data instruments and measures can be found in Appendix A: COMPASS-ND Baseline Data.
3.1.2 Key Publications
See the following key publications for protocols relating to COMPASS-ND, the online Longitudinal Online Research and Imaging System (LORIS), magnetic resonance imaging (MRI) harmonization across testing sites, and our neuropsychology test battery.
COMPASS-ND Study
Chertkow, H., Borrie, M., Whitehead, V., Black, S.E., Feldman, H.H., Gauthier, S., Hogan, D.B., Masellis, M., McGilton, K., Rockwood, K., Tierney, M.C., Andrew, M., Hsiung, G.R., Camicioli, R., Smith, E.E., Fogarty, J., Lindsay, J., Best, S., Evans, A., Das, S., Mohaddes, Z., Pilon, R., Poirier, J., Phillips, N.A., MacNamara, E., Dixon, R.A., Duchesne, S., MacKenzie, I., & Rylett, R.J. (2019). The Comprehensive Assessment of Neurodegeneration and Dementia: Canadian Cohort Study. The Canadian Journal of Neurological Sciences, 46(5), 499-511. DOI: 10.1017/cjn.2019.27. PMID: 31309917.
Data Management System (LORIS)
Mohaddes, Z., Das, S., Abou-Haidar, R., Safi-Harab, M., Blader, D., Callegaro, J., Henri-Bellemare, C., Tunteng, J.F., Evans, L., Campbell, T., Lo, D., Morin, P.E., Whitehead, V., Chertkow, H., & Evans, A.C. (2018). National Neuroinformatics Framework for Canadian Consortium on Neurodegeneration in Aging (CCNA). Frontiers in Neuroinformatics, 12, 85. DOI: 10.3389/fninf.2018.00085. PMID: 30622468; PMCID: PMC6308193.
Multisite Imaging Data Harmonization Process
Duchesne, S., Chouinard, I., Potvin, O., Fonov, V.S., Khademi, A., Bartha, R., Bellec, P., Collins, D.L., Descoteaux, M., Hoge, R., McCreary, C.R., Ramirez, J., Scott, C.J., Smith, E.E., Strother, S.C., Black, S.E., for the CIMA-Q group and the CCNA group. (2019). The Canadian Dementia Imaging Protocol: Harmonizing National Cohorts. Journal of Magnetic Resonance Imaging, 49(2), 456-465. DOI: 10.1002/jmri.26197; PMID: 30635988.
Neuropsychology Research Tests
Phillips, N.A., Fogarty, J., Pilon, R., Whitehead, V., Best, S., Di Prospero, C., Fouquet, C., Beuk, J., Mohades, Z., Das, S., Anderson, N., Belleville, S., Brambati, S., McLaughlin, P., Chertkow, H., Borrie, M. (submitted to the Canadian Journal of Aging). The Comprehensive Assessment of Neurodegeneration (COMPASS-ND) Study neuropsychology battery of the Canadian Consortium for Neurodegeneration in Aging (CCNA): Design overview and initial validation.
3.2 Data Access
CCNA is committed to maximizing the use of data by Canadian scientists. Study data are housed on LORIS, CCNA's data management platform.
3.2.1 Process for Data Access Request
- Once funded, all teams (i.e. Nominated Principal Investigators [NPI]) will obtain a LORIS account by going to: LORIS.
- Funded teams will complete a data access request which is adjudicated by the COMPASS-ND Data Access Subcommittee (DASC), a sub-committee of the CCNA Publications and Data Access Committee (PDAC). An expedited process will be implemented to review the data access request for funded teams.
- Funded teams will work with the COMPASS ND Data Access Subcommittee to ensure all project plans abide by CCNA's legal and ethical parameters. Every reasonable attempt will be made to meet the needs of those funded. Course corrections to project plans will be implemented, as appropriate, based on the outcome of this process.
3.3 Fluid Biomarkers and Biological Sample Access
Biological samples were collected on 95% of the study participants at the time of the Initial Assessment. Plans are in place to collect longitudinal samples, but this work has not yet been undertaken. Collected biosamples include blood, saliva, and urine, as well as cerebrospinal fluid (CSF) in approximately 13% of participants. Extensive, routine analyses have already been carried out on the existing samples, yielding plasma, serum, and CSF biomarkers as well as genetics. Please see Appendix B: COMPASS-ND Fluid Biomarker Data for the list of results available in the LORIS database.
In addition to running routine analyses on the collected biological materials, the study has stored samples of these materials at the Canadian Biosample Repository (Edmonton, Alberta) for future research projects. In the scope of this funding opportunity, researchers are permitted to study biomarkers beyond those measured in COMPASS-ND by undertaking the sample request process outlined below and running their own analyses.
3.3.1 Process for Fluid Biomarkers and Biological Sample Access Request
- Once funded, all teams (i.e. Nominated Principal Investigators [NPI]) will obtain a LORIS account by going to: LORIS.
- Funded teams will complete a biological sample access request on the LORIS platform. Projects will need to detail the materials required for their research and may request biological material from a reasonable number of participants up to a maximum of:
- 1.0mL for serum, plasma, urine, or saliva;
- 1 vial for buffy coat; or
- 0.5mL for CSF.
- All requests are adjudicated by the CCNA Biological Samples Access Committee (BSAC). An expedited process will be implemented to review the biological samples access request for funded teams.
- Funded teams will work with the BSAC to ensure the availability of the material and to ensure all proposed plans abide by CCNA's legal and ethical parameters.
- Every reasonable attempt will be made to meet the needs of those funded. Course corrections to project plans will be implemented, as appropriate, based on the outcome of this process.
- A material transfer agreement will apply to any approved project. The cost of sample preparation and shipping should be factored in, as needed.
4. Contact Information
If you have any questions on this Applicant Guide or on the "Canadian Consortium on Neurodegeneration in Aging (CCNA) Phase III: Research Teams" funding opportunity, you must direct all inquiries to the CIHR Contact Centre, Monday to Friday, 7:00 a.m. to 8 p.m. EDT.
CIHR Contact Centre
Telephone: 613-954-1968
Toll Free: 1-888-603-4178
E-mail: support-soutien@cihr-irsc.gc.ca
Appendix A: COMPASS-ND Baseline Data
Screening Assessment
Recruitment
- Candidate Recruitment
- General Inclusion/Exclusion Criteria
- Group-Specific Inclusion & Exclusion Criteria
- Summary and (Initial) Diagnosis
Screening Tests
All Cohorts
- Montreal Cognitive Assessment
- Subjective Cognitive Impairment & Functional Capacity
Cognitively Unimpaired (CU), Subjective Cognitive Impairment (SCI), & AD Spectrum (MCI, V-MCI, AD)Footnote 1
- Logical Memory (WMS-R)
- CERAD Word List
- Clinical Dementia Rating [CU, SCI, MCI, and V-MCI only]
- Lawton-Brody Instrumental Activities of Daily Living Scale [CU, SCI, MCI, and V-MCI only]
- Alzheimer's Disease Risk Index [CU, SCI, MCI, and V-MCI only]
FTD Spectrum (bvFTD, PPA, CBS, & PSP)Footnote 2 & LBD Spectrum (PD, PD-MCI, PDD, & LBD)Footnote 3
- Benson Complex Figure Copy
- Clinical PPA and bvFTD Features (NACC FTLD Module) [FTD spectrum only]
Mixed Dementia
- Logical Memory (WMS-R)
- CERAD Word List
- Benson Complex Figure Copy
- Clinical PPA and bvFTD Features (NACC FTLD Module)
Sociodemographic Information
- Birth; Sex; Handedness
- Language(s)
- Marital / Partner Status
- Current Living Circumstances
- Reproductive History
- Education
- Employment History
- Household Income
- Driving History
Clinical Assessment
General Health
- Biosample Collection (blood, urine, saliva, cerebrospinal fluid, buccal & fecal swabs)
- Anthropometry and Vital Signs (weight, height, heart rate, blood pressure, orthostatic change)
- Health Status and Self-Perception
- Grip Strength
- Constant Fatigue
- Basic Activities of Daily Living (from OARS Multidimensional Functional Assessment Questionnaire)
- Instrumental Activities of Daily Living (from OARS Multidimensional Functional Assessment Questionnaire)
- Physical Activity (incl. the Physical Activity Scale for the Elderly)
- Nutrition (incl. the SCREEN-II AB, Short Diet Questionnaire (CLSA))
- Oral Health
- Sleep (incl. Pittsburgh Sleep Quality Index)
- Falls and Balance (incl. Activities-specific Balance Confidence Scale)
- Gait Assessment (incl. dual-task & fast walking)
- Mayo Fluctuations Scale
- Smoking
- Alcohol Consumption
- Delirium Assessment
Psychosocial Measures
- Hobbies and Leisure Activities
- Social Network
- Social Support
- Social Activities
- Generalized Anxiety Disorder 7-Item Scale
- Geriatric Depression Scale
- Quality of Life (QoL-AD)
- End-of-Life Care
- Caregiving Assessment (incl. Zarit Burden Interview)
Sensory Measures
- Vision (incl. Mars Contrast Sensitivity Test, MNRead Acuity Charts)
- Hearing (incl. Pure Tone Audiometry, Canadian Digit Triplets Test, Hearing Handicap Inventory for the Elderly–Screening Version)
- Olfaction (Brief Smell Identification Test – Version A)
Medical and Family History
- Current Medications
- Past Medications
- Medical History
- Mental Health History
- Surgical History
- Family History
- Adverse Childhood Experiences
Disease Signs, Symptoms, and Onset
- Initial Symptoms and Disease Course
- Signs and Symptoms
- MDS – Unified Parkinson's Disease Rating Scale [LBD spectrum only]
- Parkinson's Disease Questionnaire [LBD spectrum only]
- Freezing of Gait Questionnaire [LBD spectrum only]
- Schwab & England Activities of Daily Living Scale [LBD spectrum only]
Physical and Neurological Assessment
- Physical Exam
- Neurological Exam
- Hachinski Ischaemic Score
- Clinical Diagnosis Confirmation
Primary Informant Questionnaire
- General Information
- Behavioral Inhibition Scale (NACC FTLD Module)
- Interpersonal Reactivity Index (NACC FTLD Module)
- Revised Self-Monitoring Scale (NACC FTLD Module)
- Mild Behavioral Impairment Checklist (MBI-C)
- Neuropsychiatric Inventory – Questionnaire
- Apathy Inventory
- Basic Activities of Daily Living (from OARS Multidimensional Functional Assessment Questionnaire)
- Instrumental Activities of Daily Living (from OARS Multidimensional Functional Assessment Questionnaire)
- Schwab & England Activities of Daily Living Scale [LBD spectrum only]
- Quality of Life (QoL-AD)
- Delirium Assessment
- Caregiving Assessment (incl. Zarit Burden Interview)
Neuropsychology Assessment
Premorbid IQ
- WAIS-III Vocabulary Test
Memory
- Rey Auditory Verbal Learning Test
- Brief Visuospatial Memory Test – Revised (Recall)
- CCNA–CIMA-Q Face-Name Association Task
- WAIS-III Digit Symbol – Incidental Learning
- Envelope Test
Visual Perception and Construction
- Birmingham Object Recognition Battery Object Decision Task – Easy B
- Judgment of Line Orientation Test
- Brief Visuospatial Memory Test – Revised (Copy)
Attention, Working Memory, and Processing Speed
- WAIS-III Digit Span Test
- WAIS-III Digit Symbol – Coding
Speech and Language
- D-KEFS Category Fluency Test
- Word Reading Test
- Semantic Word-Picture Matching Test
- Semantic Associates Test
- Northwestern Anagram Test (Short Form)
- Sentence Repetition Test
- Sentence Reading Test
- Noun and Verb Naming Test
- Boston Diagnostic Aphasia Exam Cookie Theft Picture Description (audio recording)
Complex Attention and Executive Function
- D-KEFS Letter Fluency Test
- Trail Making Test
- D-KEFS Color-Word Interference Test
- CCNA–CIMA-Q Sentence Inhibition Task
- CCNA Reaction Time Task
- Social Norms Questionnaire (NACC FTLD Module)
- Social Behavior Observer Checklist (NACC FTLD Module) [FTD spectrum only]
Neuroimaging Assessment
Imaging Sequences
- 3D-T1w
- FLAIR
- Dual PD/T2
- T2*
- DTI
- BOLD Resting State (fMRI)
MRI Reports
- Infarcts
- Hemorrhages
- Cortical Superficial Siderosis
- White Matter Hyperintensities (low/high burden)
- Cerebrovascular Brain Injury (low/high burden)
Volumetrics
- Hippocampus
- Entorhinal Cortex
- Globus Pallidus
- Putamen
- Caudate
- Cerebellum
- Thalamus
- Ventricles
- Total Lobar Grey Matter (GM) & White Matter (WM)
- Total GM, WM, ventricular, & intracranial volumes
Appendix B: COMPASS-ND Fluid Biomarker Data
Blood Biomarkers
General Health
- Complete blood count
- Electrolytes (Na, K, CL, bicarbonate)
- Creatinine, urea
- Liver function (AST, ALT, ALP, bilirubin)
- Vitamin B12
- Calcium
- Albumin
- 25-hydroxy vitamin D level
- Ferritin
- Glycosylated hemoglobin
- Insulin level
- Glucose
- Homocysteine
- Cystatin C
- Vascular endothelial growth factor (VEGF)
Inflammation
- Insulin like growth factor-1
- Tumour necrosis factor-alpha (TNF-α)
- Interleukin-6
- C-reactive protein
Lipidomics
- Triglycerides
- HDL cholesterol
- LDL cholesterol
- Total cholesterol
- ApoA-1
- ApoB
Synaptic Function & Plasticity
- Brain-derived neurotrophic factor (BDNF) levels
Hormone Profile
- Adrenocorticotropic Hormone (ACTH)
- Androstenedione
- Cortisol
- Dehydroepiandrosterone (DHEA) sulfate
- Dihydrotestosterone (DHT)
- Estradiol
- Estrone
- Follicle-stimulating hormone
- Free thyroxine (T4)
- Luteinizing hormone
- Progesterone
- Prolactin
- Sex hormone binding globulin (SHBG)
- Testosterone
- Thyroid stimulating hormone (TSH)
Oxidative Stress
- Vitamin E
- Alpha-1 antitrypsin
- Biliverdin
- Ferritin
SIMOA Biomarkers
- Amyloid ß 1-40 & ß 1-42
- Neurofilament light chain (NfL)
- Glial fibrillary acidic protein (GFAP)
- Phosphorylated tau 181
CSF Biomarkers
- Amyloid ß 1-42
- Total tau
- Phosphorylated tau 181
- Alpha Synuclein
Genetics
- APOE genotype
- Polygenic risk score for AD, polygenic hazard score for AD
- Polygenic risk score for PD, Polygenic hazard score for PD
- 394 identified high-interest single nucleotide polymorphisms (SNPs) that are the most related to any/all neurodegenerative diseases (complete list pending)
- Full genetics analysis (obtained from Infinium Global Diversity Array with NeuroBooster)
Microbiome
- Saliva samples collected cohort-wide are currently undergoing microbiome analyses, and these results are not currently available.
- Date modified: